C. Difficile
Latest News
Latest Videos

CME Content
More News
The impact of polymerase chain reaction (PCR) testing on patient outcomes.
Successful phase 2 trial leads to phase 3 trial towards managing C difficile.

This week, the FDA-approved antiretroviral lenacapavir as it showed treatment benefits; Oral Vancomycin Prophylaxis was explored for preventing C difficile in stem cell transplant recipients; Research into the after-effects of SARS-CoV-2 (PASC) uncovers biomarkers for post-infection sequelae; Chronic hepatitis B worsens COVID-19 outcomes, and cabotegravir ultra-long-acting (CABULA) has shown positive results.
Study dives into the complexities of using Oral Vancomycin Prophylaxis to combat Clostridioides difficile in hematopoietic stem cell transplantation recipients with insights from researcher Alexander Vartanov, MD.
The American Gastroenterological Association recommends fecal microbiota-based therapies for recurrent or severe C difficile infections and other gastrointestinal issues.

This week, increased risk of long COVID in individuals who have tested positive for the virus, advancements in the preservation of the microbiome offer new strategies for healthcare-associated infections (HAIs), FDA-approved GSK's bepirovirsen signaling the company's goal to cure viral hepatitis, CDC considers changing 5-day COVID isolation guideline, and the first victim of the Alaskapox virus highlights the critical need for increased research into emerging infectious diseases.
Advancements in microbiome preservation offer new solutions for HAI.

Study encourages enhancing protocols to clean and disinfect hospital beds and healthcare environments to stop the spread of healthcare-associated infections.
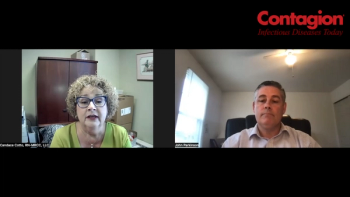
With the addition of live biotherapeutic products, a provider offers some insights into delivery and treatment considerations.

Topline data from the phase 3 study confirmed the lot consistency, immunogenicity, safety, and tolerability of a toxoid-based Clostridioides difficile infection (CDI) vaccine.

Ciprofloxacin, ceftriaxone, and piperacillin/tazobactam showed the greatest associations with adverse drug reaction case reports related to CDI.
Acurx Pharmaceuticals reported topline data from a small phase 2 study on their antibiotic, ibezapolstat, which showed it had a 96% cure rate.
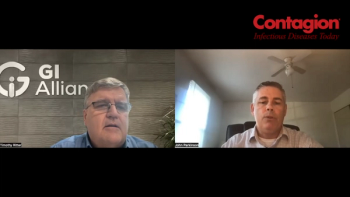
With the FDA approval of 2 of these products, which are indicated for recurrent Clostridioides difficile infection (CDI), a study reviewed which types of clinicians are prescribing them and the protocols in using them.

With the addition of 2 new live biotherapeutic products, a clinician reviews the application, storage and administration techniques for them.

New data showed an approximate 60% success rate of recurrent C difficile prevention in adults with comorbid conditions including CKD and cardiac disease.

New phase 1b data supports further investigation into Adiso Therapeutic's prospective treatment, ADS024.
This market has seen a flurry of activity, as shown by a review of the latest agents.

The patient advocacy organization has events in place and is ready to launch its annual C diff awareness campaign.

This class of medications were associated with a greater risk of Clostridioides difficile infection (CDI).
Company plans to start recruiting for its REPREVE study this year.
Excess death risks from GI diseases, above pre-pandemic patterns, were largest for C difficile colitis, followed by GI hemorrhage, ulcers, and colorectal cancer.

Investigators explored the makeup of antibiotic resistant genes after administration of the live biotherapeutic product, Rebyota (fecal microbiota, Live-jslm), to see what differences would be discovered.

In this week's infectious disease news, the Novavax COVID-19 booster gets authorized in the US; Moderna reports topline data on its influenza-COVID-19 combination vaccine; the differences between live biotherapeutic products and conventional FMTs; and the influence of alcohol on hepatitis C treatment.
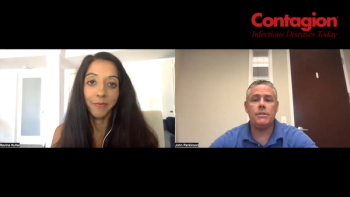
Without regulations for donation practices for conventional fecal microbiota transplants (FMT), clinicians need to consider which therapeutic agents for recurrent C difficile treatment to utilize.
Screening for Clostridioides difficile in all admissions to ICU identified asymptomatic carriers and their risk for developing and/or transmitting infection.