Zoonotic & Vector-Borne Diseases
Latest News

Video Series

Latest Videos
Shorts



CME Content
More News

TNX-4800 targets OspA to block Borrelia transmission; positive phase 1 data reported and an adaptive phase 2/3 study is planned.

CDC’s recent ArboNET snapshot shows widespread human and non-human activity, preliminary totals subject to change, and no lineage reporting on the dashboard.

In the second interview segment with leaders from UNC's Special Pathogen Center, they discuss what it is like to be in the field during an outbreak caring for patients with high-consequence infectious diseases, and offer insights on how well prepared the US is for these situations.

Jonathan Parr, MD, MPH, discusses his research about this deadly and burdensome disease and how he and his team identify these parasitic strains with a combination of epidemiologic field work and the latest laboratory technology.
CDC encourages travelers to take enhanced precautions, get vaccinated, and prevent mosquito bites in affected areas, with special warnings for pregnant individuals.

Regulators cite four new serious adverse event reports, halting US distribution while global access efforts continue.

ECDC warns climate shifts are driving longer mosquito seasons and expanding disease transmission, calling for stronger surveillance, vector control, and clinician preparedness.

Morris County resident was infected despite no recent travel; risk to public remains low, officials say.

Pediatric infectious disease expert Sharon Nachman, MD, explains how to identify symptoms, assess travel risk, and prevent mosquito-borne illness in kids.
After a three-month review, the FDA and EMA have cleared continued use of IXCHIQ in adults 60+, adding new safety warnings for elderly individuals with chronic conditions.

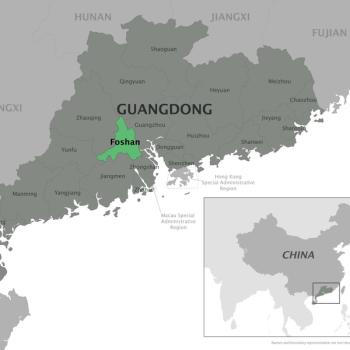
China Reports Over 7,000 Chikungunya Cases in Guangdong Province Amid Aggressive Containment Efforts
China's largest recorded chikungunya outbreak prompts strict mosquito control and mandatory hospitalization in Foshan.

Two deaths and eight infections in Italy highlight the need for enhanced surveillance, proactive vector control, and cross-border collaboration in Europe and beyond.

At ASM Microbe 2025, Pelumi Oladipo discusses E marmotae’s reduced motility, misidentification as E coli, and the diagnostic tools closing the gap.


Climate-driven changes and other drivers of the expanding geographic range of Ixodes ticks and Lyme disease are explored.

Significant short-term impact of MDA with dihydroartemisinin–piperaquine plus primaquine in a moderate-to-low transmission setting, highlighting the need for full seasonal coverage and community engagement to sustain gains.

Centers for Disease Control and Prevention report underscores need for improved surveillance, donor screening, and public awareness as rare WNV strain emerges and Powassan virus cases hit new high.

Ep 1, Part 4 of 4, Robert C Bransfield, MD, continues to share how vector-borne infections may trigger psychiatric symptoms not through direct brain infection, but by disrupting immune signaling and gene expression.

Ep 1, Part 3 of 4 with Robert Bransfield, MD, explores treatment-resistant mental health issues linked to infection history and diverse progression patterns.

Evaluating safety and efficacy against symptomatic, virologically confirmed dengue in children and adolescents.

Andrew Handel, MD, discusses prevention, how to remove ticks, and the burden of Lyme disease in certain populations.

Valneva’s immunization, Ixchiq, was well tolerated in children ages 1 to 11 years regardless of the dose or previous chikungunya infection.

Jyothi N Purushotham, PhD, and Holly L Lutz, PhD, call for integrated immunological and ecological strategies to counter fast-evolving viral threats.

Alexander Laurenson presents T-cell-focused tool at the 2025 Vaccinology Conference using data from 18 countries and 748 HLA alleles to address immune diversity in Africa.

A small percentage of people who contract the tick-borne illness have lingering symptoms and health issues after the acute infection has been treated, but there are no FDA-approved therapies to deal with prolonged symptoms. A new report looks to address this issue.