Vaccines
Latest News
Latest Videos

CME Content
More News

With the release of these guidelines and the US Health and Human Services canceling the mid-March FDA VRBPAC meeting to discuss these vaccine recommendations, Robert Hopkins Jr, MD, the medical director of the National Foundation for Infectious Diseases (NFID), offers insight on these developments.

The interim results demonstrated a slightly better vaccine effectiveness in individuals who are immunocompetent vs immunocompromised individuals.

The most recent CDC report accounts for 33 million flu illnesses, 430,000 hospitalizations, and 19,000 deaths this flu season, with Influenza A(H1N1) and A(H3N2) as the primary strains.

On the heels of the first death in an ongoing US-based measles outbreak, Paul Offit, MD, discusses how outbreaks are the first sign that childhood vaccination rates are starting to decline.

Tina Tan, MD, FIDSA, FPIDS, FAAP, Infectious Diseases Society of America (IDSA) president discusses the changes and offers a glimpse of what the US can expect in terms of limited access to new vaccines, increased incidence rates of disease, and new public health policy regarding immunizations.

The World Health Organization (WHO) reports on the vaccine uptake in that region, and the Global Polio Eradication Initiative reports that some countries recording new cases.

Jeff Freiberg, MD, PhD, on the rising emerging infection concerns of dengue, mpox, measles, and H5N1 influenza in clinical practice.

A new study published in JAMA Network Open points out that immunization also occurred in individuals who did not receive routine prenatal or infant vaccines.
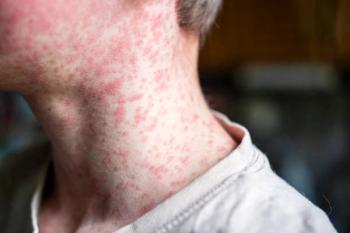
Confirmed measles cases in Texas have risen from 24 to 58 in just six days, with 13 hospitalizations reported.

The company's trivalent vaccine (mRNA-1403) was being studied in a phase 3 trial.

In a statement, the official, Ralph Abraham, MD, along with the state’s deputy surgeon general, wrote that because of “COVID missteps” they will no longer encourage vaccination to people.

In the Phase 3 trial, GSK's vaccine met all 11 primary endpoints, providing expanded options for preventing invasive meningococcal disease in individuals aged 10-25 years.

The approval is based on positive phase 3 clinical trial results, offering an effective option for travelers and others at risk of chikungunya, the mosquito-borne illness.

Su H Wang, MD, FACP, MPH discusses combating vaccine misinformation, preventing outbreaks, and highlighting global health interconnectedness

Heather Platt, MD, discusses Merck’s latest achievement with Capvaxive and its potential to significantly reduce invasive pneumococcal disease and pneumonia in high-risk adult populations across Europe.

Robert Walker, MD discussed the company's application for full licensure and the importance of vaccination.

Vaccines and treatments are up for approval in early to mid-2025, including a chikungunya vaccine, a meningococcal vaccine, a monoclonal antibody for RSV, and more.

In a small trial, the Hecolin hepatitis E vaccine demonstrated further utility outside of previous study parameters.

The Phase 1 clinical trial for ARCT-2304, a self-amplifying mRNA vaccine, seeks to address pandemic influenza risk as H5N1 cases increase.

As politics increasingly intersects with science, infectious disease clinicians are called to take on advocacy roles. By engaging with their communities, clinicians can dispel misconceptions, highlight the importance of federal health institutions, and emphasize their role in safeguarding public health and advancing medical progress.

The continuation of misinformation and disinformation being disseminated in the public is causing more public health communication issues as mistruths associate the vaccines with cancer.

The federal agency approved safety labeling changes to the prescribing information for Abrysvo (Pfizer) and Arexvy (GSK) RSV vaccines for seniors after data from clinical trials, reports to the Vaccine Adverse Event Reporting System (VAERS), and a postmarketing study.

The updated recommendations simplify vaccine eligibility while addressing both cost-effectiveness and racial health disparities.

Investigators evaluated strategies to simplify the vaccination process to yield higher rates of adult immunization, similar to that of the COVID-19 pandemic.

Reactogenicity of mRNA COVID-19 with inactivated influenza vaccine was similar whether given at same time or separated by 1-2 weeks.