Vaccines
Latest News
Latest Videos

CME Content
More News

Matthew Hepburn, MD, offers some insights on his time as vaccine development lead for Operation Warp Speed and the ability to develop a COVID-19 vaccine within a year.

Novavax COVID-19 Vaccine Linked to Fewer Adverse Effects in Health Care Workers and First Responders
At ESCMID Global 2025, SHIELD study data show Novavax recipients experienced fewer adverse effects and missed less work compared with those who received the Pfizer-BioNTech COVID-19 vaccine.

After delays in the review of the COVID-19 vaccine—which missed its April 1 review deadline—the agency has requested new data.

The University of Minnesota’s Center for Infectious Disease Research and Policy (CIDRAP) announced its initiative that will look to ensure reliable vaccine data and information is available to the public and medical community.

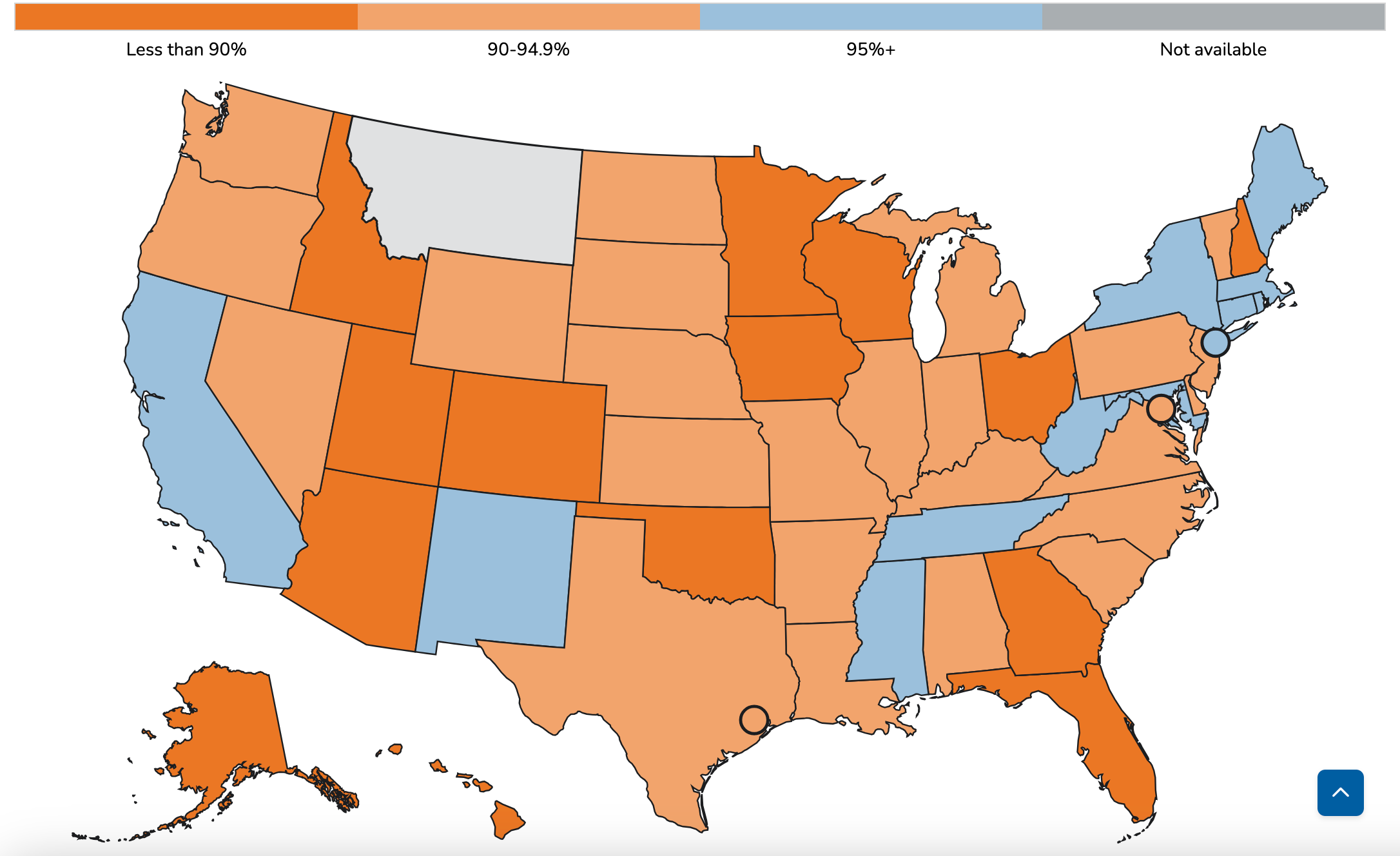
Ninety-four percent of cases were tied to 10 outbreaks, Texas leading with 624 cases, while Michigan and Montana reported the first outbreaks since 2019 and 1990, respectively.
Study of 400,000 pediatric records shows vaccine’s effectiveness in preventing Long COVID during Delta and Omicron waves.

ACIP provides recommendations on meningococcal vaccines, RSV vaccination for at-risk adults, and chikungunya vaccines for travelers and laboratory workers, while also addressing safety concerns and potential expansions of use for each.
CDC maintains low public health risk despite more cases, the first reported death, and accelerated vaccine development to enhance pandemic preparedness.

With the Trump administration’s plans to cease funding for Gavi, the Vaccine Alliance, as well as to reduce the US Department of Health and Human Services personnel, public health, both internationally and domestically, is going to be widely affected.

A child who was not vaccinated succumbed to measles pulmonary failure. Additionally, increased cases and hospitalizations continue to grow greatly in the state.

Novavax’s COVID-19 vaccine, Merck’s RSV antibody, and Innoviva’s gonorrhea antibiotic await regulatory decisions this quarter.

New 21-valent conjugate vaccine authorized to combat invasive pneumococcal disease and pneumonia in individuals aged 18 and older.

In the second installment of our interview with Robert Hopkins Jr, MD, the medical director of the National Foundation for Infectious Diseases (NFID), he discusses some of the takeaways in how public health messaging was lost on the public, which lead to mistrust, and thus leaving open the door for disinformation and misinformation.

David Wohl, MD, explains the health risks of visceral fat and the role of treatments like Tesamorelin F8 in managing the condition.

Heather Platt, MD, discusses Merck’s ongoing research into real-world effectiveness and cost-effectiveness and offers a preview of data to be presented at ESCMID 2025.

In the second installment of our COVID-19 pandemic series, Robert Hopkins Jr, MD, the medical director of the National Foundation for Infectious Diseases (NFID), discusses the paradox that has arisen from the COVID-19 vaccines that in spite of their development in record time, what remains is a deeper mistrust in immunizations overall.

Onyema Ogbuagu, MBBCh, FACP, FIDSA discussed 96% virologic suppression with lenacapavir-based therapy, showing similar results to daily oral regimens.
The vaccine is based on an attenuated rabies vaccine that was subsequently inactivated to make the vaccine candidate. The National Institutes of Health (NIH) is sponsoring the trial.
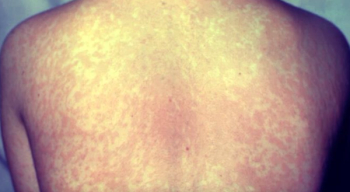
Cases in the US continue to increase, and a new analysis shows 2024 was the highest year for measles cases in Europe in 25 years.

Phase 1 data on VH184, a third-generation integrase strand transfer inhibitor, and VH499, a novel HIV-1 capsid inhibitor, highlighting their antiviral potency, safety profiles, and potential for long-acting injectable formulations.

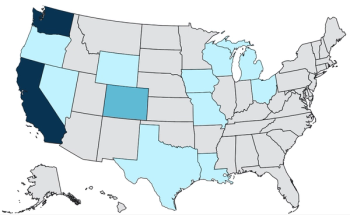
Mia Moore, PhD, presents findings showing 74% reduction in hospitalizations and key insights into primary vaccination and booster impact on population immunity in Washington and Oregon.

New pharmacokinetic data, shared by Moupali Das, MD, MPH, reveal long-lasting plasma concentrations, surpassing twice-yearly subcutaneous formulation for HIV prevention

Phase 1b trial data demonstrated immune response, with 85% reduction in viral shedding and robust mucosal immunity in elderly adults.

The phase 1/2a trial of BioNTech's BNT165e vaccine, involving nearly 180 participants, faces delays as global malaria control efforts struggle with funding and political uncertainties.

Approved vaccines for chikungunya, meningococcal disease, updates on HIV prevention, key regulatory decisions, and more.