For providers on the front lines, lessons learned can help inform strategies to enhance the protection of their health going forward.

For providers on the front lines, lessons learned can help inform strategies to enhance the protection of their health going forward.
Changes in microbiology and antimicrobial resistance patterns warrant reevaluation of our current therapies for this condition.
Clinicians can make room for infrequent treatment options as patients enjoy their daily lives.
New booster shots, antigen testing, and a shrinking incubation period: these were the top COVID-19 stories of 2022.
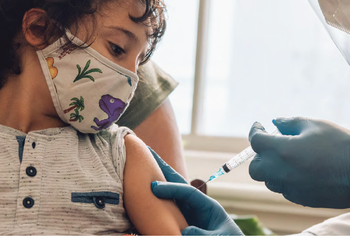
Vaccinations and mitigations for COVID-19 and other respiratory viruses have not been made a priority for young children. Let’s change this before the next big surge hits.
Cefepimetaniborbactam may represent a safe and effective carbapenemsparing agent in cUTI and AP, whereas ridinilazole may play an important role in treating CDI.

A new monoclonal antibody, invasive group A strep, and genital herpes topped the list of this week's most-read stories.
The new recommendation from the organization believes it will help avoid delays in patient care.
This study was one of the largest to address the use of IV ceftriaxone in the context of MSSA bacteremia compared with cefazolin.
Virax Biolabs has developed a T-cell testing platform to develop an immune risk profile against viral threats.

This therapy is the first of a new class of antiretrovirals, and it is indicated for people with multi-drug resistance, intolerance, or safety considerations.

Telemedicine use is likely to continue expanding even as the COVID-19 pandemic slowly resolves.

Roche’s Actemra is the first FDA approved monoclonal antibody to be used for COVID-19.

The committee will meet on January 26 to discuss whether and how the composition for primary doses of the currently available COVID-19 vaccines should be modified.

Editor-in-Chief Jason Gallagher discusses the surging number of cases of influenza and RSV while there is a concomitant shortage of amoxicillin suspension.

A central portal to support clinical practice for patients with or at risk for HIV was launched.

In this mini-podcast, infectious diseases clinical pharmacy specialist Bruce M. Jones, PharmD, FIDSA, BCPS, breaks down the “very straightforward criteria” he uses to decide whether a skin and soft tissue infection (SSTI) patient should be inpatient or outpatient.
This pathogen is the third most frequently isolated nontuberculous mycobacteria seen in the United States. Here is a review of how it presents and treatment options.
Genital herpes (HSV-2) affects quality-of-life but also causes substantial economic losses, especially in low- and middle-income countries.

The New Drug Application (NDA) for the company’s investigational therapy, Olorofim, looks to serve an unmet medical need in this treatment space.
The Centers for Disease Control and Prevention (CDC) is looking into the possibility of this rare group of infections emerging.

2 years after the COVID-19 vaccine, we're focusing on who is still getting the most severe disease and why.

A new CDC report highlights another health-related area where interruptions occurred due to the pandemic, but data suggests both improved rapidly.

Europe’s Committee for Medicinal Products for Human Use (CHMP) issued a positive opinion for Triumeq PD, the first dispersible single tablet regimen with dolutegravir, for children living with HIV.

TP-05, a novel, oral therapeutic recently reported topline results from its phase 1 study.
With these findings, the Uppsala University investigators believe loss of the Y chromosome in white blood cells can predict which patients are at high risk of severe COVID-19 disease progression.

A large health system saw a reduction of 40% and 70% respectively when the antiviral was prescribed.
A single-dose of acoziborole could simplify the treatment of this fatal infection.

A new report looked at mortality over a 2 ½ year period, and the epidemiology of who was most affected.

In this podcast episode, Christian Sandrock, MD, MPH, FCCP, describes the need to make significant progress in antimicrobial stewardship.