The Los Angeles County Department of Public Health updated the public on the specific local case and reminds everyone of the importance of vaccination to protect the community.

The Los Angeles County Department of Public Health updated the public on the specific local case and reminds everyone of the importance of vaccination to protect the community.

Democratic Republic of the Congo reports 28 suspected cases and 15 deaths, as Africa CDC and WHO mobilize vaccines, surveillance, and rapid response teams to contain the 16th Ebola outbreak since 1976.

Daniela Weiskopf, PhD, of the La Jolla Institute for Immunology, discusses her research in this area including the unique findings and pathways for therapeutics.

This week, US measles totals reached 1,454, a 750-plus-day SARS-CoV-2 infection in a person with HIV informed care, implementation science efforts advanced access, and a universal flu adjuvant showed promise.

CDC’s recent ArboNET snapshot shows widespread human and non-human activity, preliminary totals subject to change, and no lineage reporting on the dashboard.

Merck reported the results for its phase 3 trial for its vaccine, Capvaxive, which was found to be noninferior to the pneumococcal 23-valent polysaccharide vaccine (PPSV23) for each of the 12 serotypes shared between the vaccines.

Record-high 127.1/100,000 hospitalizations with co-circulating influenza A strains and similar in-hospital severity to prior years.
Head-to-head phase 1 data indicate larger fecal IgA responses after a single tablet vs first-generation constructs, with plans to advance to mid-stage testing.

In the second installment of an interview with Jacinda Abdul-Mutakabbir, PharmD, MPH, she continues the discussion around limited to no health care engagement due to these factors, and the importance of translating data from the bench to bedside when looking at disease and infection prevention.

Oxford’s Prof Katrina Lythgoe and Mahan Ghafari, DPhil, report ONS-CIS data on 576 persistent UK infections with rapid within-host evolution and rare transmission after one month.

Corner Therapeutics says its "hyperactivator" adjuvant technology can offer protection against all virus strains.
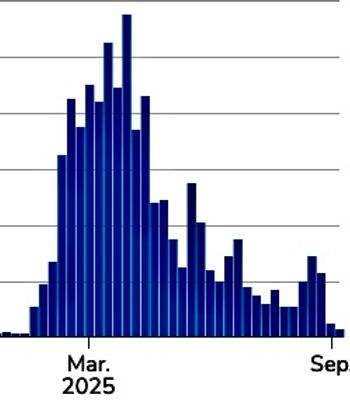
CDC reports 3 new cases this past week, bringing the 2025 total to 1,454 confirmed cases across 42 jurisdictions, with 37 outbreaks.

Jacinda Abdul-Mutakabbir, PharmD, MPH, provides insights on her recently published study on this topic and how the 2 are connected.

William Hanage, PhD, discusses viral evolution, transmission risk, and clinical implications for immunocompromised patients.

Pranita Tamma, MD, MHS, continues her conversation about a study she was involved in that compared ceftolozane-tazobactam and ceftazidime-avibactam. She discusses how an analysis of infection source and treatment patterns found no evidence that combination therapy improves outcomes in patients with drug-resistant Pseudomonas, consistent with prior studies and clinical trials.

16 cases of Salmonella Enteritidis across 10 states, including seven hospitalizations, are under investigation; antimicrobial resistance may complicate treatment.

Study finds 12 antibodies with potent neutralizing activity against multiple SARS-CoV-2 strains, including Delta and BA5.

Cocrystal Pharma to launch phase 1b study by year-end 2025, targeting prevention and treatment of viral gastroenteritis caused by norovirus.

Michael Herce, MD, MPH, MSCR, discusses this concept and how it works to improve access to healthcare for populations who have been disproportionately burdened by various infectious diseases such as HIV, tuberculosis, and COVID-19.

Bluejay Therapeutics’ investigational therapy, brelovitug, offers weekly self-administered injections and will be studied against the only approved treatment outside the US.

41 cases of nongroupable N meningitidis conjunctivitis were identified at Joint Base San Antonio-Lackland, highlighting diagnostic, treatment, and surveillance implications for congregate living settings.

From COVID-19 antivirals to RSV, influenza, malaria, and gram-negative infections, new research highlights both advances and ongoing challenges in infectious disease prevention and treatment.

Up to 2 million people in low- and lower-middle-income countries may gain access to the long-acting PrEP option under a three-year, no-profit agreement.

In the second interview segment with leaders from UNC's Special Pathogen Center, they discuss what it is like to be in the field during an outbreak caring for patients with high-consequence infectious diseases, and offer insights on how well prepared the US is for these situations.

In the midst of many changes surrounding aminoglycosides over the past several years, clinicians may be left wondering if these agents still have a role in modern clinical practice. This article summarizes changes impacting aminoglycosides, discusses where these agents may still have a role in the context of gram-negative infections, and explores future areas of study.

David Margolis, MD discusses his team’s work at UNC looking at combination treatment that includes the cancer drug, vorinostat, and immunotherapy as a potential HIV cure.

The financing supports phase 3 evaluation of Sidiprev, a first-in-class, nonantibiotic therapy designed to prevent C difficile infections, a leading US health care–associated threat.
Compared to culture/sensitivity results (C/S) results, ICU-based PCR testing of hospital-acquired pneumonia was associated with lower treatment costs and better antibiotic stewardship, but not higher cure rates.

WHO and HRP highlight new research on HIV communication, STI priorities, and inclusive strategies to strengthen sexual health worldwide.

This becomes the first state in the United States to do so, and of course, this decision will affect the childhood vaccine schedule in Florida. Infectious Diseases Society of America (IDSA) President, Tina Tan, MD, weighs in on this decision and what this could mean for an increase of vaccine-preventable diseases and who would be vulnerable to them.