
On the same day WHO Director-General Tedros Adhanom Ghebreyesus declared the end of the COVID-19 public health emergency, CDC Director Rochelle Walensky announced she will be stepping down at the end of June.

On the same day WHO Director-General Tedros Adhanom Ghebreyesus declared the end of the COVID-19 public health emergency, CDC Director Rochelle Walensky announced she will be stepping down at the end of June.

Epidemiologists report cluster of pediatric intracranial abscess in Clark County, Nevada with Streptococcus intermedius the most common isolate.

Compared to fidaxomicin and vancomycinfecal, fecal microbiota transplant (FMT) was the most cost-effective treatment for first and subsequent recurrent C difficile infection.

RSV expert Steven Varga, PhD, describes the failed vaccine trials that paved the way for the world's first respiratory syncytial virus (RSV) vaccine, as well as the innovations still needed to protect vulnerable populations against RSV infection.

Outpatient parenteral antimicrobial therapy (OPAT) offers benefits for patients as well as providing cost effectiveness; however, it is not without its challenges. Two clinicians provide insights on this modality.

A clinician offers some insights and considerations on clinical care as it pertains to climate.

The FDA has authorized GSK’s Arexvy (RSVPreF3 +AS01E) prevent lower respiratory tract disease (LRTD) caused by RSV in adults 60 years and older.

COVID-19 symptom or viral rebound in the absence of antiviral treatment is common, but the combination of symptom and viral relapse is rare.
A novel oral microbial therapy under development by Vedanta Biosciences reduced the risk of recurrence of Clostridioides difficile infection in a phase 2 clinical trial.

Decreased racial and ethnic disparities in COVID-19 mortalities between the first and Omicron waves of the pandemic could be explained by increased deaths in non-Hispanic White adults and changes in the geographic spread of the pandemic.

The Gates Medical Research Institute (MRI) is collaborating with Atreca on an investigational therapy, MAM01, which is moving into clinic trials this year.
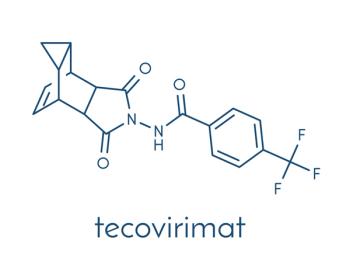
Among mpox patients treated with tecovirimat, there was no difference in treatment outcomes between those living with HIV and those without HIV.

The WHO issued its first ever list of priority antibiotics for pediatrics to encourage research and development targeting needs of that population.
The vaccine efficacy of the Novavax’s NVX-CoV2373 was 79.5% in adolescents.

Members of both branches brought it back to gain support and passage of a bill aimed at greater development of antibiotics.

Rates of central line–associated bloodstream infection (CLABSI), catheter-associated urinary tract infection (CAUTI), and methicillin-resistant Staphylococcus aureus (MRSA) bacteremia all increased in hospitalized COVID-19 patients.

The complexity of dealing with emerging infectious diseases makes preparedness difficult.

A clinician offers ideas about who to target for immunization and strategic considerations when having these conversations.

One of the most challenging aspects of clinicans’ jobs is giving patients and staff bad news. A clinician offers some strategies to help shape an approach in having these conversations.

Artificial intelligence (AI) is being utilized already says one clinician. She explains her optimism about the technology, and says it will help augment any potential challenges providers have now or in the future.

At 7.1%, vitamin C deficiency in the US is rare. Unfortunately, identifying scurvy is complex, frustrating, and time-consuming for both patient and provider.

A paradigm shift is emerging when it comes to the clinical approach to prescribing antibiotics. One clinician weighs in on using this class of therapeutics more judiciously.

An epidemiologist offers some insights on what we need to take into a potential next pandemic including infrastructure investment and more effectively communicating messages to the public.
Panel moderator Rodney Rohde, PhD, notes that word choice is important and clinicians should emphasize the COVID-19 vaccine is not a live virus but is instead designed to challenge the immune system to protect against the virus.
Containing 20 serotypes, Pfizer's Prevnar 20 grants the broadest serotype coverage of any pediatric pneumococcal conjugate vaccine.
An outbreak of the highly infectious measles virus has led American Samoa to declare a public health emergency.

This gathering is the nation’s largest for internal medicine doctors and has many infectious disease-related scientific sessions.

By addressing ART-related medication errors and increasing linkage to care, antiretroviral stewardship programs can improve management of inpatients with HIV.

Vowst (SER-109) has received FDA approval to treat recurrent C diff infection, making it the first oral microbiome therapeutic.
The findings of this study could pave the way for new, disease-targeted probiotics.