
Antibiotics
Latest News

Petition Urges EPA to Halt Antibiotic and Antifungal Pesticide Use Over Resistance Risks
Latest Videos

CME Content
More News

A DOXYVAC substudy found higher rates of tetM-mediated tetracycline resistance and more isolates with decreased cefixime susceptibility among MSM using doxycycline PEP.

Fewer people are falling ill with TB, and fewer with drug-resistant illness according to the 2025 WHO Global Tuberculosis Report.

David Cameron, PhD, discusses how researchers discovered its antibiotic, Debio 1453, through structure-guided design and advanced it from potent preclinical activity to first-in-human testing.

AMR Action Fund CEO Henry Skinner, PhD, MBE, discusses the significance of this week, provides an update on the PASTEUR Act, and explains the differing approaches between Europe and the US with regard to push-pull incentives.
The Leapfrog Group has released its Fall 2025 Hospital Safety Grades showing states such as Utah and New Jersey remain in the top 5 of rankings. Data on health systems is also included in the report.

Rachel Britt, PharmD, BCIDP, provides insights on an evolving understanding of the long-time antibiotic’s limitations for these infections and provides guidance on other agents and the role of diagnostics in finding the best agents for patients.

PBPs are more than static β lactam targets. Host conditions rewire PBP activity and peptidoglycan architecture, shaping tolerance, resistance, and how we design salvage regimens.

The federal agency gave the nod to Meitheal Pharmaceuticals for its antibiotic, Contepo, an intravenous fosfomycin offering a new mechanism of action and a new option for adults with complicated urinary tract infections, including those caused by resistant gram-negative pathogens.

The Global Antimicrobial Resistance and Use Surveillance System (GLASS) of the WHO finds antibiotic resistance and the particular threat of resistant Gram-negative bacteria disproportionately prevalent in low- and middle-income countries. Here is part 1 of 2-part coverage of the WHO Global Antibiotic Resistance Surveillance Report.

A new genomic study reveals that up to 18% of urinary tract infections (UTIs) in Southern California may stem from E coli strains transmitted through contaminated poultry and meat, disproportionately affecting residents of low-income neighborhoods.

Veronique Dartois, PhD, and Thomas Dick, PhD, discuss the multidrug resistance associated with non-TB mycobacteria and their research, which focuses on developing a novel version of this class of antibiotics for these infections.

Barry Kreiswirth, PhD, discusses how taking an approach similar to that for streptococcal pneumonia can be applied to a potential Klebsiella vaccine.

John Osiecki, PhD, explains how faster, standardized testing can speed detection and strengthen antimicrobial stewardship.

Cefiderocol is noninferior to standard of care empiric treatment of noscomial Gram-negative bloodstream infections in open-label "Game-Changer" trial.

The Center for Discovery and Innovation’s Barry Kreiswirth, PhD, discusses this emerging challenge that is ongoing in both the community and healthcare settings, especially around resistant urinary tract infections and how this type of resistance can grow exponentially across strain and pathogens.

AI predicted mechanism of action for investigational, narrow spectrum antibiotic against pathogens in Crohn's disease and IBD-related conditions.

The company is advancing R327G, a novel synthetic anti-infective for diabetic foot infections, through a phase 3 clinical trial in Indonesia, marking a major step toward the first new anti-infective class approval in over 40 years.

Despite modest progress, Staphylococcus aureus bloodstream infections—particularly those caused by MRSA—remain a major cause of morbidity and mortality, underscoring the urgent need for novel, broad-spectrum treatments such as ceftobiprole that can address both resistant and susceptible strains.
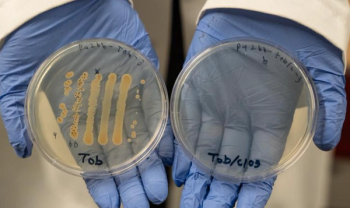
University of Oregon researchers found that adding a common chemical, chlorate, to standard antibiotics made them up to 10,000 times more effective in lab tests against hard-to-treat bacteria, offering a potential breakthrough for chronic wound care.

Infections caused by NDM-producing carbapenem-resistant Enterobacterales have risen more than 4-fold since 2019, raising alarms about limited treatment options, delayed detection, and heightened risks of transmission and mortality in health care settings. TAXIS Pharmaceuticals Chief Scientific Officer Ajit Parhi, PhD, offers some insights on NDM-CRE, antimicrobial resistance, and an overview of his company’s pipeline.

Recce Pharmaceuticals has begun its study for this indication in Indonesia with its investigational product, RECCE 327 topical gel (R327G).

Stewardship programs that reduce initial use of extended-spectrum antibiotics appear to influence antibiotic choice throughout hospitalization.

William Schaffner, MD, reviews clinical workup, treatment, and preventative measurements.

The 2025 ATS guidelines for community-acquired pneumonia update recommendations on imaging, antibiotic use, treatment duration, and corticosteroid use, emphasizing lung ultrasound as an alternative diagnostic tool, more selective empiric antibiotic prescribing, shorter courses of antibiotics for stable patients, and limited use of corticosteroids to severe inpatient cases.

Jennifer Le, PharmD, MAS, FIDSA, FCCP, FCSHP, discusses her collaboration on this innovative work with this therapy in severely ill children.









































































































































