
Research set to be presented next month found that people with resolved COVID-19, and in some cases active COVID-19, could safely donate organs.

Research set to be presented next month found that people with resolved COVID-19, and in some cases active COVID-19, could safely donate organs.

Along with reporting positive data in the youngest pediatric population, the company is working with the Food and Drug Administration (FDA) for emergency use authorization of its vaccine in children 6 years to under 11 years as well as the 12-17 age groups.

COVID-19 vaccines were less protective against symptomatic infection from the Omicron COVID-19 variant than the Delta variant.

Here’s a roundup of popular foods and products that were recalled this week by the US Food and Drug Administration (FDA).

Series-specific antibiotics can be far less effective if there are other microbes present.

The Omicron variant of SARS-CoV-2 had a secondary attack rate of 25.1% in Norwegian households compared with 19.4% for the Delta variant.

Nearly 8 in 10 patients with recurrent C. diff. Infection were successfully treated after up to 2 doses of the microbiota-based therapy.

A matched cohort study found COVID-19 infection increased the risk of new type 2 diabetes diagnosis. Compared to patients with acute upper respiratory tract infections, COVID-19 patients were 28% more likely to develop diabetes.

There are many unknowns about the BA.2 COVID-19 variant. It appears 50-60% more transmissible than the original Omicron variant, but health experts are mixed on whether cases will spike dramatically.

The study authors wrote that few people who initiate tenofovir disoproxil fumarate-based oral PrEP rarely experience clinically significant kidney impairment.

A Texas study found children and adolescents who previously contracted COVID-19 retained protective antibodies for 6 or more months after infection. However, natural infection plus vaccination remains the best defense against COVID-19.

People who use drugs face obstacles, like homelessness and stigma, that hinder their access to healthcare. Dr. Brianna Norton opened the Montefiore-NYHRE Clinic, a first-of its kind academic medical center, to serve marginalized people holistically and respectfully.
Check out these important stories we covered this past week.
A new virtual program aims to train clinicians and other providers on terminology and how to engage patients in effective dialogue and counseling.

The submission is for a fourth dose of its Spikevax (mRNA-1273) vaccine in adults 18 years of age and older.

Policies keep case counts down even when vaccination rates are low, new analysis finds.
A study of over 8 million participants found no correlation between COVID-19 vaccination and developing neurological conditions. However, a risk of some neurological conditions was increased after COVID-19 infection.

A recent JAMA article discussed the role of caregiver vaccination status in pediatric hospitals and ethics of exclusion.
A Tufts University research time identified a novel testing method to detect Lyme disease reinfection and successful treatment.

Engaging in antimicrobial stewardship programs led to a reduction in antibiotic usage in long-term care facilities, potentially reducing the growing risk of antibiotic resistance.

Pfizer’s COVID-19 pill Paxlovid, which includes nirmatrelvir and ritonavir, reduced the risk of hospitalization and death by 89% among adults at high risk for severe disease, recently published phase 2/3 trial data show.

The study authors suggest further research and investment to identify areas of support for individuals at the highest risk of poor outcomes.

The application is based on 2 Israeli studies for the additional shot for people 65 years and older.

In children and adolescents, the Pfizer-BioNTech vaccine was only mildly effective against symptomatic and asymptomatic COVID-19 infections. Broken down by variant, Omicron infections were more likely to occur and more likely to be asymptomatic.
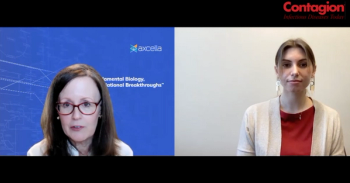
Axcella Therapeutics chief medical officer Dr. Margaret Koziel discusses the stage 2a clinical trial for their novel long covid treatment.

This study comes just weeks after the company announced another HIV vaccine trial.

In a case series of 18 patients, all but 3 were cured by single-donor fecal microbiota transplantation capsule therapy.
The new COVID-19 sub-variant, called “Deltacron” or “Stealth Omicron,” appears to be the most contagious strain yet. However, this does not necessarily mean there will be a dramatic spike in cases.

In a nationally televised interview, Pfizer Albert Bourla said it is necessary to fend off waning protection.

Findings of JAMA Network Open analysis reveal effects of interventions designed to prevent spread.