
The target action date for the V920 investigational Ebola vaccine is set for March 14, 2020.

The target action date for the V920 investigational Ebola vaccine is set for March 14, 2020.

Being geographically close a measles outbreak could change the way individuals feel about getting vaccinated against the disease.

As the summer comes to a close, our editorial team is looking back at the top FDA approvals of 2019 to date and looking ahead at what is left on the docket this year.

Investigators with Seqirus presented findings from a handful of studies on a MF59 adjuvanted influenza vaccine at the Options for the Control of Influenza (OPTIONS X) Conference in Singapore.
The crux of new information centers around the vaccine virus composition for the 2019-2020 season, and recent labeling changes for previously licensed vaccines.
The African country was to be the third participant in a pilot program that would’ve inoculated 360,000 children against the mosquito-borne virus.
Texas could be the site of the next large measles outbreak in the United States, according to a new study that highlights the low vaccination rates among schools there.
No one knows exactly why thousands of patients in dozens of states have contracted hepatitis A in recent years. In the absence of an explanation, public health officials are focusing on vaccination.
The Biomedical Advanced Research and Development Authority will contribute $23 million for Merck to continue manufacturing the Ebola vaccine over the next year.

Three children in US Customs and Border Protection custody have died since December 2018, partially due to influenza. Now, the infectious disease community is rallying for vaccination for migrants in detention facilities.
William Schaffner, MD, breaks down the new ACIP guideline recommendation, the pediatric benefit of PCV13 which drove the decision, and means by which it can improve clinical decisions surrounding vaccination.

Parents seeking medical exclusions for their children must now have their physician fill out a state form explaining specifically why the exemption is warranted.
As outbreaks of vaccine-preventable diseases grow, it is important to review vaccination recommendations by age group.

Investigators in the first human trial of a MERS-CoV candidate report strong immune responses and no serious adverse events, advancing the vaccine candidate to phase 1/2a and phase 2 trials.

An update for clinical providers.
The Ad26.ZEBOV, MVA-BN-Filo vaccine regimen will be evaluated in an open-label, single-arm study of 800 participants, including frontline health care workers.
The first step (prime) is to immunize with conventional vaccines through a needle in the arm. The second step (pull), is to apply an antibiotic cream that helps attract, T cells, to the site of virus growth.

The latest guidance on preparing for and responding to measles in your health care facility.

When speaking with patients or parents, it is important for health care practitioners to stress that vaccines are safe, and vaccines save lives.
The adjusted hazard ratios for 1-dose and 2-dose recipients were comparable to the 3-dose recipients (1 dose 1.01 [95%CI 0.81–1.26], 2 doses 1.00 [0.85–1.17]) after adjusting for age at vaccination among the vaccinated group.
The phase 2a study compares a bivalent combination of Clade C and Mosaic gp140 with a single-valent Clade C gp140.
Several states have or are considering laws that would circumvent consent from anti-vax parents.

Mosaico plans to register 3800 HIV-negative participants aged 18 to 60 years when enrollment opens later this year.
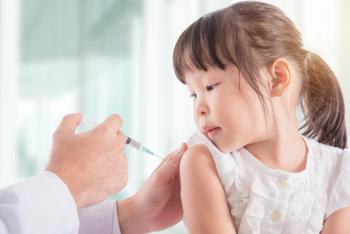
Following the elimination of personal belief exemptions, the statewide rate of kindergartners without up-to-date status for required vaccinations decreased from 9.84% in 2013 to 4.87% in 2017.

ACIP voted 10-to-4 to expand the recommended HPV vaccine “catch-up�” age for men from 21 to 26, matching the existing guidelines for women.