The European Commission has approved their first dengue vaccine, Takeda’s TAK-003, as climate change and urbanization contribute to a worldwide rise in dengue incidence.
Nina Cosdon is the associate editor for Contagion. Before joining MJH Life Sciences, she graduated magna cum laude from Denison University in 2021 with a degree in Communication. You can find her reading, hiking, or antiquing, or by emailing her at ncosdon@mjhlifesciences.com.

The European Commission has approved their first dengue vaccine, Takeda’s TAK-003, as climate change and urbanization contribute to a worldwide rise in dengue incidence.

Ferring Chief Scientific Officer Dr. Elizabeth Garner explains the FDA approval of recurrent C diff treatment RBX2660 provides more than a treatment option, it’s a promise of safety and standardization.

COVID-19 hospital patients were prescribed 21.81% more antibiotics than patients without COVID-19. How did this affect their risk of coinfection?

Today, Pfizer-BioNTech filed submitted an Emergency Use Authorization (EUA) to administer their Omicron BA.4/BA.5-adapted bivalent COVID-19 vaccine booster in children under 5 years of age.

With major updates in HIV, C difficile, hepatitis, yeast infections, and skin and soft tissue infections, this week's Infectious Disease Update has something for everyone.
A germline-targeting HIV vaccine candidate elicited broadly neutralizing antibodies in 97% of recipients.

Ibrexafungerp tablets (Brexafemme) were already FDA approved to treat vulvovaginal candidiasis (VVC) and have now received a second indication for the reduction of recurrent VVC (yeast infection).

Tonight, the FDA announced that Rebyota (RBX2660) is the first approved fecal microbiota product, intended to prevent recurrent C difficile infection in adults.
Takeda’s investigational dengue vaccine TAK-003 is currently under evaluation for preventing any dengue virus serotype in individuals 4-60 years old.
November is C difficile Awareness Month. We're recapping the most significant potential therapies and remaining challenges from the past month.

Dr. Teena Chopra discusses the global need to do more to prevent healthcare-associated infections like C difficile in vulnerable patient populations.

New clinical findings suggest the Pfizer-BioNTech bivalent booster elicited more neutralizing antibody titers for all tested emerging Omicron sublineages, compared to their original COVID-19 vaccine.

Read and watch the infectious disease trial data shared this week.

Dr. Tosin Goje explains the promise of ibrexafungerp for clearing yeast infection and improving quality of life for these patients.
Concerns of injectable HIV PrEP safety and efficacy demonstrate a need for health care providers to share reliable information with at-risk populations.

Although HIV PrEP uptake is gradually increasing, usage remains low among key at-risk populations.

Young women who binge drink, and especially those who use multiple substances, had a higher risk of COVID-19 infection and mental health complications.

What are the characteristics and demographics of the chronic hepatitis B patients who develop severe outcomes?

Looking for the latest developments in infectious disease? Here are the top stories Contagion covered this week.

In Boston school districts, lifting mask mandates was correlated with an additional 44.9 COVID-19 infections per 1000 students and staffers.
HIV PrEP prescriptions increased from 0 among people who use injection drugs, but overall PrEP uptake remains low.

Initially, 28-29% of patients with chronic hepatitis B achieved undetectable virus levels after 24 weeks of treatment with GSK’s bepirovirsen, but this dropped to 9-10% of patients during a phase 2b trial.

Studies suggest young women’s substance use is catching up to men's. Tammy Chung, PhD, examined whether this was exacerbated by the COVID-19 pandemic.

The intranasal, live-attenuated vaccine CodaVax-RSV was granted FDA Fast Track designation. Clinical trials are expected to launch soon.

Experts discuss a range of the most topical infectious disease research, presented at the recent IDWeek 2022 conference.

Previously thought to be difficult or impossible, one study found HCV and drug use can be treated concurrently in young adults.

Dengue is the most prevalent and important mosquito-borne virus in the world. Atea Pharmaceuticals presented positive data for their direct-acting dengue antiviral, AT-752.

How do we increase HIV PrEP uptake? “I think the biggest takeaway here is that people need options,” said Travis Sanchez, DVM, MPH.
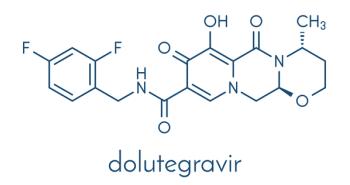
Pregnant people taking dolutegravir were more likely to achieve HIV viral suppression and less likely to have a preterm birth than participants using other forms of ART.

Is it safe and beneficial to administer a second immunomodulator in patients with moderate COVID-19? How do we identify which patients are likely to progress to severe disease?