Articles by Contagion Editorial Team

Initial findings from the phase 1 CLARITY study offer the first direct comparison of the acceptability and tolerability of single-dose cabotegravir and lenacapavir long-acting injections in HIV-negative adults, revealing differences in patient and health care provider preferences.

This week, learn about the Center for Discovery and Innovation's research around antivirals, the hepatitis C bill in Congress, how AI is influencing antimicrobial development, and more.

The company is advancing R327G, a novel synthetic anti-infective for diabetic foot infections, through a phase 3 clinical trial in Indonesia, marking a major step toward the first new anti-infective class approval in over 40 years.

The US Department of Agriculture’s Food Safety and Inspection Service (FSIS) provides information on the 2 companies recalls and the products.

This week, there are C diff and hepatitis roundup reports, how COVID-19 has disrupted traditional respiratory virus patterns with summer surges now preceding the typical winter influenza season, a review of a trial comparing dalbavancin with standard intravenous therapy for Staphylococcus aureus, and more.
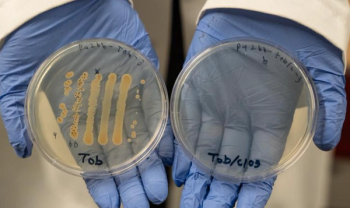
University of Oregon researchers found that adding a common chemical, chlorate, to standard antibiotics made them up to 10,000 times more effective in lab tests against hard-to-treat bacteria, offering a potential breakthrough for chronic wound care.

Infections caused by NDM-producing carbapenem-resistant Enterobacterales have risen more than 4-fold since 2019, raising alarms about limited treatment options, delayed detection, and heightened risks of transmission and mortality in health care settings. TAXIS Pharmaceuticals Chief Scientific Officer Ajit Parhi, PhD, offers some insights on NDM-CRE, antimicrobial resistance, and an overview of his company’s pipeline.

A June–September roundup spanning hepatitis research and surveillance data, FDA and ACIP policy moves, diet and diagnostics insights, and pipeline updates from screening innovations to HBV/HDV/HCV trials and approvals.

A June–September C diff roundup spanning diagnostics and stewardship debates, microbiome-based therapies and FMT optimization, prevention trials and funding, real-world outcomes, and persistent gaps in clinician practice and public awareness.

This week, review of ACIP’s latest decisions, analysis links food insecurity with higher long COVID risk, and advocacy for immune-informed antibiotic development with updated susceptibility testing.

Although the number of people with knowledge about the infection rose, the results demonstrate a continued need for further education and awareness among the public.

This week, OPAT for gram-negative infections expands to outpatients amid infusion complexity and stability limits, HHS and CDC add five ACIP members days before meeting, and more.

Corner Therapeutics says its "hyperactivator" adjuvant technology can offer protection against all virus strains.

Study finds 12 antibodies with potent neutralizing activity against multiple SARS-CoV-2 strains, including Delta and BA5.

Bluejay Therapeutics’ investigational therapy, brelovitug, offers weekly self-administered injections and will be studied against the only approved treatment outside the US.

Iterum Therapeutics has launched its sulopenem etzadroxil and probenecid (Orlynvah), which is the first and only oral penem antibiotic in the US.

New authorization limits shots to adults ≥65 and individuals with underlying health conditions, with ACIP set to review guidance and insurance coverage implications.

From chikungunya setbacks to COVID-19 booster guidance and childhood vaccine safety initiatives, here are the top regulatory and research developments this summer.
The American College of Cardiology is providing recommendations on the influenza, COVID-19, RSV vaccinations and others.



The designation is for the company’s TXA14007, its investigational antibiotic to be studied in combination with levofloxacin.

Full committee removal raises concerns over vaccine policy stability and scientific independence.

This week, FDA approved Moderna’s improved COVID-19 vaccine mRNA, MAD-ID findings showed omadacycline’s 86% success rate in treating infections in immunocompromised patients, consistent safety in preventing recurrent CDI in patients with comorbidities, and more.

Valneva’s immunization, Ixchiq, was well tolerated in children ages 1 to 11 years regardless of the dose or previous chikungunya infection.
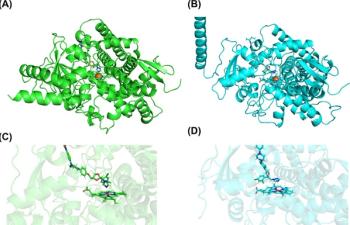
A published study reveals how an extra gene allows a specific mycetoma-causing fungus to neutralize itraconazole when treating the neglected tropical disease.

Innoviva launches ceftobiprole in the US, shifts in FDA COVID-19 vaccine policy, a surge in fungal infections, and more.

A Centers for Disease Control and Prevention (CDC) publication reports on REPEXH01, a strain of Shiga toxin–producing Escherichia coli (STEC) O157:H7, which has been responsible for at least 9 outbreaks in the US since 2017.

The company detailed its preliminary 24-week post–end-of-treatment data for its tobevibart and elebsiran combination, which is being studied for chronic hepatitis B.

A study of an H5N1 strain from a Michigan dairy worker showed airborne transmission in ferrets, bulevirtide achieved sustained virologic response in chronic hepatitis D patients, and more.


























