The findings of this study could pave the way for new, disease-targeted probiotics.
Important clinical considerations to know about resistant CMV infections.

The last week in April is World Immunization Week, and we're recapping the most recent and significant developments in infectious disease vaccines.

The study authors wanted to further understand post-COVID-19 infection “brain fog” and sought a new name for the phenomenon.

The antiviral is being studied for treatment for those at high-risk for severe COVID-19, no matter their vaccine status.

The key is using the right dose on the right patient at the right time.
This study surveyed parents’ opinions on the risks of COVID-19 infection versus vaccination to determine how they affected the decision to vaccinate a child against COVID-19.
The combination appears more likely than either agent alone to reduce risk of early COVID-19 symptoms worsening and requiring hospitalization.

Data from New Haven County, Connecticut, suggests the pandemic might have hurt efforts to curb recurrent CDI.

This was the first study to compare clinical outcomes in carbapenemase-producing (CP-CRE) and non–carbapenemase-producing (nCP-CRE) infections.

The floods, heat waves, droughts, and other calamities caused by excessive greenhouse gas emissions also make us more vulnerable to ill effects of pathogens such as bacteria, viruses, plants and fungi.
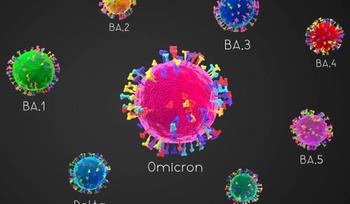
Omicron lineages, and especially BA.5, were determined to have higher reinfection rates and lower disease severity than previously circulating variants of concern.

The Peggy Lillis Foundation will host its summit on Monday and has a number of speakers who will discuss topics related to this healthcare-associated infection (HAI).

Two RSV vaccines for seniors have completed phase 3 trials and demonstrated beneficial results. FDA decisions on potential approvals for both could be forthcoming in the next few weeks.
While efforts to curb in-hospital infection seem to have paid off, community-acquired cases appear headed in the opposite direction.

The CDC and FDA now recommend the Moderna and Pfizer-BioNTech mRNA bivalent COVID-19 vaccines be used for all vaccinations in the US.

The 340B program has had a great impact on access to HIV treatment and prevention services in the US.

Patients who contracted COVID-19 later in the pandemic (2021-2022) were more likely to develop new chronic diseases after infection than patients who caught COVID-19 in 2020.
A key stakeholder offers his insights on the importance of the new Biden plan to eliminate hepatitis C as well as the federal strategy to get more people linkage to care for hepatitis B.

Anemia is both common and independently associated with poor clinical outcomes in respiratory infections, including COVID-19.

Increased understanding of HCV protective immunity and HCV envelope glycoprotein structure and function may propel development of an HCV vaccine.
The FDA moved to harmonize primary and booster COVID-19 vaccine doses, deciding only the Moderna and Pfizer-BioNTech bivalent mRNA vaccines should be administered to individuals 6 months and older.

The FDA’s Antimicrobial Drugs Advisory Committee (AMDAC) voted 12-0 in favor of recommending this antibiotic and sets up a PDUFA target action date of May 29.
Since the lifting of public health restrictions, the country saw a sizable increase in incidence rates.

Post-COVID-19 conditions were more common in unvaccinated children than in children who had received at least 1 dose of a COVID-19 vaccine.

With an FDA approval, it would be the first new class of oral antibiotics for uncomplicated urinary tract infections (uUTI) in over 20 years.

Poor sleep quality, deterioration in sleep quality, and sleep regularity were all linked to impaired lung function.

Several years after its FDA approval, this antibiotic continues to prove its efficacy in vitro across various pathogens related to these infections.

Alita Miller, PhD, senior vice president of research at Entasis Therapeutics, discusses positive trial data for Sulbactam-durlobactam (SUL-DUR) slated to be presented at ECCMID 2023.