Please join advocates for prevention to celebrate the miracles the liver performs in maintaining the bodies’ systems, and ways to increase education to the public about the organ.

Please join advocates for prevention to celebrate the miracles the liver performs in maintaining the bodies’ systems, and ways to increase education to the public about the organ.

The streptococcal pneumoniae urine antigen (SPUA) test was studied over the course of a year to determine if it provided any benefits in terms of diagnostic capability or aided in reducing empirical antibiotic use.
A study presented at the Society for Healthcare Epidemiology of America (SHEA) conference assesses the link between antibiotic use and C difficile infection in Tennessee.

An analysis of NHANES data looked at HCV infection and risk of prostate cancer, and found contrary evidence from previous research.
Men5CV is expected to target 5 strains of the meningococcus bacteria, reducing meningitis cases across the region.

A large cohort study found this patient group more susceptible to repeat admissions, and points to potential issues associated with ambulatory care.
The results were presented at the Society for Healthcare Epidemiology of America (SHEA) conference, highlighting this first documented case of C auris moving from an adult to a pediatric unit within the state.

In these patient encounters, a deep cough as a symptom raised concerns around pneumonia and may have prompted prescription of antimicrobials, but did not show a resolution of symptoms sooner.
A study, presented at the Society for Healthcare Epidemiology of America (SHEA) conference, assesses laxative administration before CDI testing in Veteran Affairs (VA) hospitals and explores the demographic and contextual factors with non-adherence to guidelines.

Hospitals grapple with the costly burden of healthcare-associated infections (HAIs), and artificial intelligence (AI) is emerging as a game-changer in revolutionizing infection prevention strategies.

Comparative analysis of SARS-CoV-2 infection across pediatrics, adults, and older adults.

The company’s investigational ABCWY vaccine candidate will be reviewed by the FDA by February 14, 2025.
Current progress of WHO's strategies to enhance global health outcomes through improved diagnosis and treatment of viral hepatitis.

A small study found physical health problems lingered 1 year after their hospital stay.

FDA notified of allergen threat by New Haven food manufacturer due to mislabeled products.
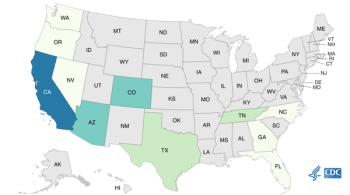
Officials announce an end of Listeria traced to Rizo-López dairy products involving queso fresco and cotija.

Incidence of hospital-onset Clostridioides difficile infection differed between hospitals that implemented the CDC prevention strategies and those that did not, but not necessarily because of the strategies.

The Centers for Disease Control and Prevention (CDC) and Detroit Health Department stress the importance of vaccination and surveillance.

B virus case involves a man who had contact with wild monkeys, FDA approves therapy for teens living with HIV, FDA grants EUA for Flu A/B and COVID at-home testing, and more this week from Contagion.

Danjuma Adda on WHO's efforts against Hepatitis B and his call for global vaccination and treatment efforts.
New data demonstrates protection against new variants.

In a new federal survey, as many as 3 in 10 US adults have had the condition at some point.
Study addresses the racial and ethnic inequalities in TB incidence rates.

This annual event hosted by The Peggy Lillis Foundation (PLF) released their full agenda for advocacy and education that will be happen next week in Washington DC.
Study evaluates the outcomes of orthotopic liver transplantation of HIV-HBV coinfected patients.
New data shows young children are less likely to get diagnosed and get into the continuum of care including getting prescribed antiretroviral therapy (ART).
Water sampling reveals COVID-19 trends in homeless populations.
A large trial supports de-escalation from empiric, broad-spectrum antibiotic treatment for Enterobacterales bacteremia to a non-antipseudomonal agent.

The CDC's take on the importance of HIV awareness and testing among our youth.
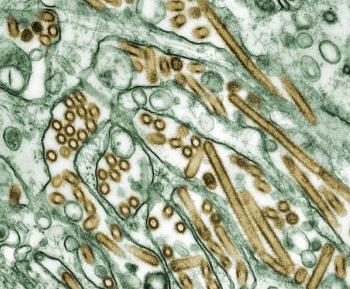
The origins of the recent H5N1 (avian flu) case in Texas remain unknown, but a researcher offers insights into transmission between animals and humans, the likelihood of more cases, vaccine availability, and treatment.