Salmonella becomes more dangerous as antimicrobial resistance grows. Read about its most common manifestations.

Salmonella becomes more dangerous as antimicrobial resistance grows. Read about its most common manifestations.

Although there are challenges for clinical and infectious disease pharmacists when trying to apply this concept, here are some considerations and strategies to employ stewardship in this setting.

Progress in rapid diagnostic and point-of-care testing propelled by the pandemic can improve timeliness and precision of anti-infective treatment.
However, according to one study the risk of heart inflammation increases with age and amplifies in patients with hepatitis C (HCV) compared to patients without HCV.

The Vaccines and Related Biological Products Advisory Committee's (VRBPAC) positive vote follows the committee’s review of the data from the biologics license application (BLA) for RBX2660.

This will include 3 trials to examine the therapy in different formulations in HIV studies.
Eravacycline is an antibiotic that has been touted for possible treatment of difficult-to-treat resistant (DTR) Gram-negative infections. But can it tackle carbapenem-resistant Acinetobacter baumannii (CRAB) infections?

The study authors identified four main approaches to antimicrobial stewardship programs after questionnaire and focus group discussions with ASP leaders.
The Paxlovid approval reaffirms the importance of pharmacists in fighting COVID-19.

As greenhouse gas emissions result in floods, heat waves, and more extreme weather events, people and pathogens are thrown closer together.
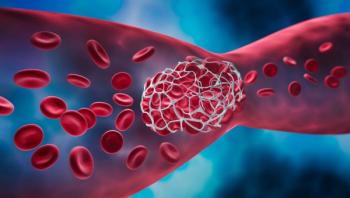
In 2020 alone, the COVID-19 pandemic may have caused an additional 10500 heart attacks, strokes, and other blood clot complications across England and Wales.
Here is a review of using this therapy in this patient population.

After positive top-line data from phase 3 trials for their meningococcal vaccine, MenABCWY, Pfizer intends to submit a Biologics License Application (BLA) to the FDA.
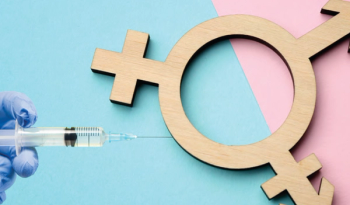
Gender-affirming hormone therapy (GAHT) therapy consists of administration of exogenous hormones and suppression of endogenous hormone production, with the goal of obtaining characteristics more congruent with an individual’s gender identity. Here is a review of these therapies and consideration for PrEP and the risks associated with HIV in this diverse population.
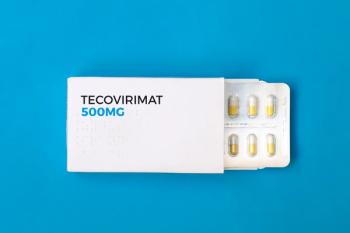
The AIDS Clinical Trials Group (ACTG) is launching a phase 3 trial to evaluate the safety and efficacy of tecovirimat to treat monkeypox in vulnerable populations.

The vaccine is available in adults 18 years and older.

The Lancet Commission called for global cooperation to end the continued spread of COVID-19 and effectively prevent future pandemics.
Why do some children develop polio-like paralysis after contracting enterovirus? Here’s everything we know about the rare but increasing reports of acute flaccid myelitis.

There are a number of programs designed to help lower rates of infection and transmission amongst this industry's workforce in South Africa.

The declaration not only puts the country on a list of countries circulating the viruses, but it also means polio continues to be transmitted in Rockland County, NY, and surrounding areas.
COVID-19 viral load rebound occurred in 2.3% of nirmatrelvir–ritonavir (Paxlovid) recipients and in 1.7% of placebo recipients.

A meta-analysis study shows having the comorbidity presents significant health risks for this patient population.

The brand’s specific meat product may contain E coli O157:H7.

Adenovirus was determined to have caused most cases of hepatitis of unknown origin in children.
Investigators surmise a natural bat exposure within certain areas of the world may correspond with lower COVID-19 incidence rates.

Injected COVID-19 mRNA vaccines alone were ineffective at producing mucosal antibody responses, according to a recent study that suggested seeking new strategies such as intranasal vaccines.

Investigators noted 2 distinct phases of HIV virus levels, meaning any infected cells that survive treatment can become latent, only to reignite HIV infection after antiretroviral therapy is stopped.

The same 5-year survival rates with kidney transplants from HCV positive and negative donors supports revising HCV "penalty" in ranking donors.

Texas judge Reed O’Connor ruled that requiring employers to cover the medical expense of HIV PrEP is a violation of religious freedom.