
We’ve rounded up a list of important US Food and Drug Administration (FDA) and US Department of Agriculture (USDA) recalls from this past week.

We’ve rounded up a list of important US Food and Drug Administration (FDA) and US Department of Agriculture (USDA) recalls from this past week.

Stay up-to-date on the latest infectious disease news by checking out our top 5 articles of the week.
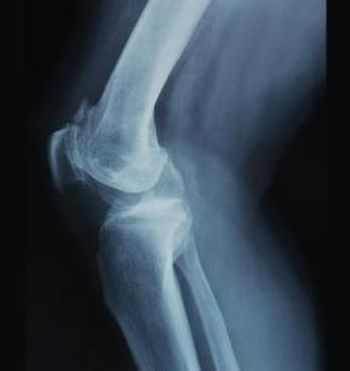
A new analysis of more than half a million knee replacements finds patient-specific factors and certain surgical decisions can make a difference in the rate of infections.

An infamous beauty procedure is causing concern over spa safety failures after 2 clients were diagnosed with HIV.

The final results of the PARTNER study have been published in the journal The Lancet and confirm that an undetectable viral load on HIV treatment renders an individual sexually non-infectious.

Difficulties connecting with physicians mean not all people at risk of acquiring HIV have access to PrEP. Putting nurses on the front lines could change that.
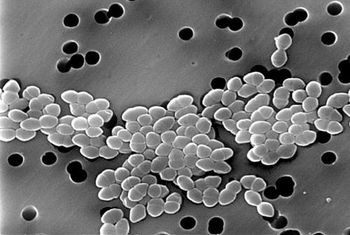
The CDC considers VRE to be a “concerning threat,” and estimates that the bug sickens some 20,000 Americans annually, causing death in roughly 1300 people per year.
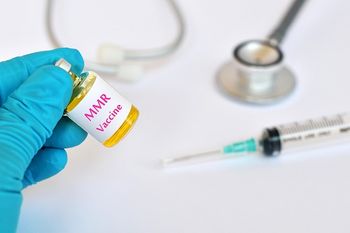
Merck, the sole supplier of the MMR vaccine in the United States, is increasing production to meet growing demand from areas where measles outbreaks are ongoing.
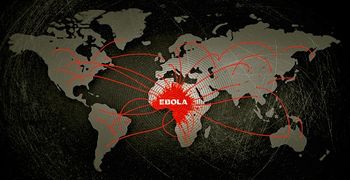
As Ebola case counts continue to climb in the ongoing outbreak, emergency preparedness simulations are underway in Uganda.

A third dose of the measles-mumps-rubella vaccine may be a safe and effective response to concerns about waning immunity to mumps among young adults.

The FDA has approved the Dengvaxia vaccine for the prevention of dengue disease in children 9 to 16 years who have laboratory-confirmed previous dengue infection.

Designated frontline hospitals were expected to identify, isolate, and hold an Ebola patient for 12-24 hours, but an analysis cites gaps in biopreparedness in these facilities.
Surgical-site infection was not found to be associated with duration of prophylaxis, but adjusted odds of AKI and C diff infection increased with each additional day of antimicrobial exposure.
“60 Minutes” becomes the latest media outlet to cover antibiotic resistance, but are they giving the whole story?

Updated guidelines on the treatment and management of HIV in children and adolescents reflect new data on antiretroviral drugs as well as a call for mental health screenings.

The CRL requests that Nabriva address issues related to facility inspections and manufacturing deficiencies at one of Nabriva’s contract manufacturers prior to the FDA approving the New Drug Application.

The FDA has approved Mavyret (glecaprevir and pibrentasvir) tablets to treat all 6 genotypes of hepatitis C in children 12 to 17 years.

An investigation by Harvard T.H. Chan School of Public Health found that 27% of sampled e-cigarettes contained traces of endotoxin while 81% contained traces of glucan.

Here are the most recent recommendations for these tricky fungal infections in solid organ transplant recipients.

A 21-week-long flu season begins to wind down while investigators find that flu infections in consecutive seasons are more likely in young children.

Experts from the fields of microbiology and infectious disease share their biggest takeaways and highlights from ECCMID 2019.

Nicole Cotroneo, BS, discusses her research presented at ECCMID 2019 on the activity of tebipenem against multidrug-resistant urinary tract infection-causing pathogens.

From spring conferences to recently declared food-borne outbreaks, April was a busy month full of infectious disease news.

Timothy Minogue, PhD, discusses his presentation on miRNAs for Ebola and the capabilities of the host profiling side and the pathogen-derived side.

A team of investigators is examining why some transgender women taking hormones may need higher levels of PrEP medication.
The CDC reports 704 cases of measles have been confirmed to date since January 1, 2019, across 22 states.

Experts from the fields of microbiology and infectious disease share their biggest takeaways and highlights from ECCMID 2019.
The growth of opioid addiction in the United States has led to a significant convergence between substance use disorders and infectious diseases. Physicians who specialize in the latter need to take note.

An intervention involving improved communication and antibiotic timeouts in nursing homes reduced the days of antibiotic therapy, according to a recent study.

Here is a look at infectious disease-related US Food and Drug Administration news from the week of April 21, 2019.